Where are we going with this? This page will assist in developing the ability to describe, classify, and give examples of various kinds of reactions: synthesis (i.e., combination), decomposition, single displacement, double displacement, acid/base, and combustion.
Combustion
Fire! Heat! Oxygen!
Fire! Heat! Oxygen!
When oxygen reacts with other substances to create energy in the form of heat and light.
Something burns by combining with oxygen.
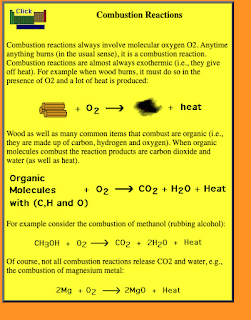
"Many combustion reactions occur with a hydrocarbon, a compound made up solely of carbon and hydrogen. The products of the combustion of hydrocarbons are always carbon dioxide and water. Many hydrocarbons are used as fuel because their combustion releases very large amount of heat energy. Propane (C3H8) is a gaseous hydrocarbon that is commonly used as the fuel source in gas grills."
(See For More)
(See For More)
 |
| For More Info |
It is not unusual to think only of reactions producing CO2 and H2O as combustion. This is typically what is thought of when thinking of combustion: some hydrocarbon burning to produce CO2 and H2O.
However, inorganic combustion reactions also exist.
 |
| See For More Info |
With inorganic combustion, the production of CO2 and H2O will not be present.
- In combustion, one of the reactants must be oxygen.
- Energy is produced in the form of light and/or heat.
- Not all combustion reactions are fast; some occur slowly.
There are many examples of combustion, such as:
2H2 + O2 --> 2H2O
2Mg + O2 --> 2MgO
No comments:
Post a Comment